Categories
- ADHESIVE DRESSINGS
- ADHESIVE PLASTERS
- ADVANCED WOUND CARE DRESSINGS
- AIDS TO DAILY LIVING
- AIRWAY MANAGEMENT
- ANAESTHETIC MASKS
- ANTI EMBOLISM COMPRESSION DVT STOCKINGS
- ANTISEPTICS
- APRONS
- BANDAGES
- BED PROTECTORS
- BEST SELLERS
- BIOPSY PUNCHES & CURETTES
- BLOOD COLLECTION ACCESSORIES
- BLOOD PRESSURE MONITORS
- BODY WIPES
- BRACES
- BURN PRODUCTS
- CANNULAS
- CAPS
- CATHETERISATION ACCESSORIES
- CLEARANCE - SALE ITEMS
- COMBINE DRESSINGS
- CONTINENCE CARE
- COTTON APPLICATORS
- COVERALLS
- CPR DEVICES
- DEFIBRILLATORS
- DENTAL INSTRUMENTS
- DIAGNOSTIC EQUIPMENT
- DISSECTING KITS
- DRESSING PACKS
- ELECTRODES
- ENDOTRACHEAL TUBES
- ENTERAL FEEDING
- EXTENSION SETS
- EYE & WOUND IRRIGATION
- EYE/EAR WEAR
- FINGER COTS
- FIRST AID EQUIPMENT
- FIRST AID KITS
- FIRST AID SUPPLIES
- FLUIDS
- FORCEPS
- GAUZES
- GELS & CREAMS
- GIVING SETS
- GLOVES
- GOWNS
- GUEDELS
- HAND SANITISERS
- HOLLOWWARE
- HOT & COLD THERAPY
- INSTRUMENT PACKS
- INTRAVENOUS CARE
- IRRIGATION
- IV ACCESSORIES
- LARYNGEAL MASK AIRWAYS
- LARYNGOSCOPES
- LUBRICANTS
- MASKS
- MOBILITY
- NASOPHARYNGEAL
- NEEDLE HOLDERS
- NEEDLES
- NEW ARRIVALS
- NON-ADHERENT PADS
- NURSES EQUIPMENT
- OPHTHALMOSCOPES
- OTHER
- OTOSCOPES
- OXYGEN THERAPY
- PEN LIGHTS
- PROBES
- PROTECTIVE GARMENTS
- Protective Underwear
- PULSE OXIMETERS
- RAZORS
- RECORDING CHART PAPER
- RESPIRATORY / RESUSCITATION
- RESPIRATORY DEVICES
- RESUSCITATORS
- ROLLATORS
- SCALPELS & BLADES
- SCISSORS
- SHARPS CONTAINERS
- SHOE COVERS
- SHOWER
- SKIN & SURFACE PREPS
- SLEEVE PROTECTORS
- SPECULAS
- SPRAYS
- STERILISATION POUCHES
- STETHOSCOPES
- SUCTION TUBING
- SURFACE WIPES
- SURGICAL INSTRUMENTS
- SURGICAL PENS
- SUTURE KITS
- SUTURES
- SWABS
- SYRINGES
- TAPES
- THERMOMETERS
- TOILET
- TOURNIQUETS
- TOWELS
- TUBING CLAMPS
- ULTRASOUND GEL
- URINE BAGS
- URINE CATHETERS
- URINE COLLECTORS
- WALKING STICKS
- WHEELCHAIRS
- WINGED INFUSION SETS
- WOUND CARE
- WOUND PADS
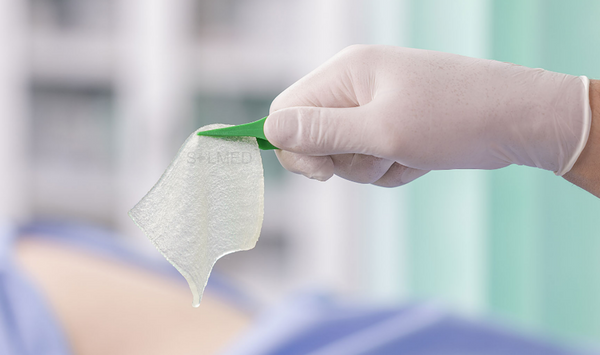
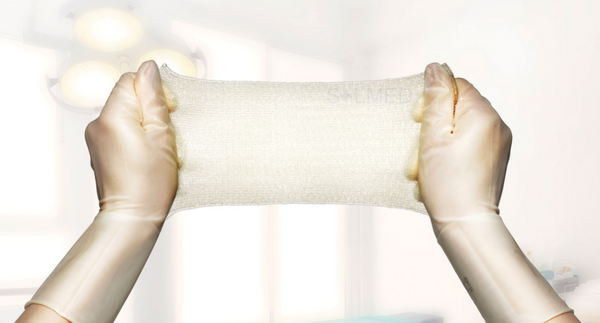
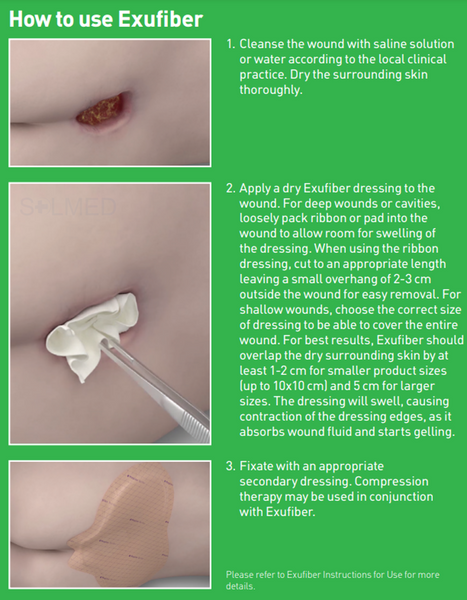
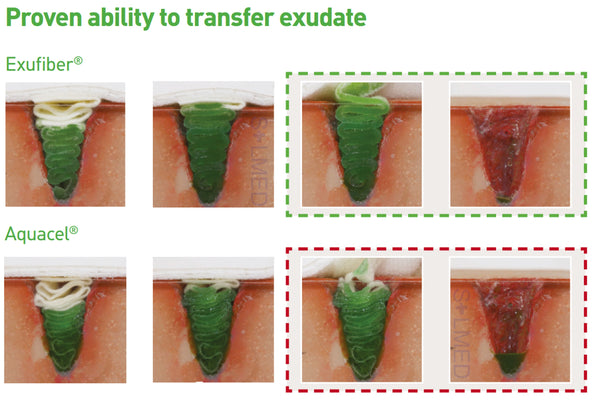
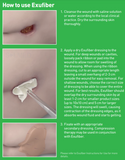
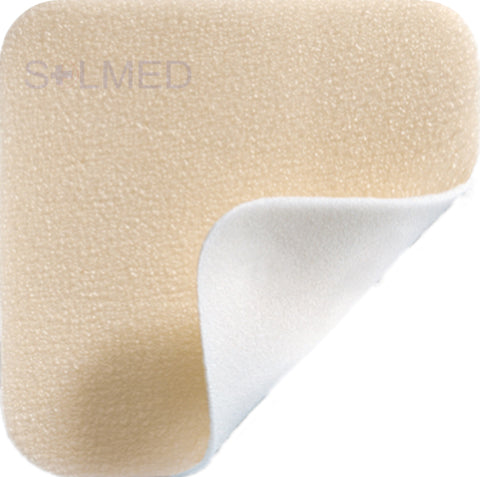
Exufiber is a sterile non-woven dressing made from highly absorbent polyvinyl alcohol fibres. In contact with wound exudate, Exufiber transforms into a gel that facilitates moist wound healing and ease of removal during dressing changes. Exufiber absorbs and retains wound exudate.
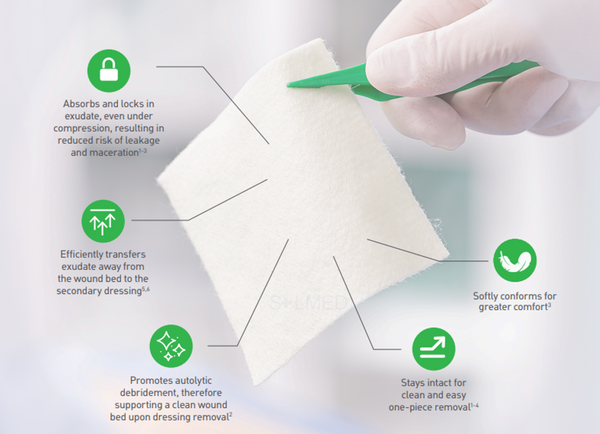
Benefits of Exufiber
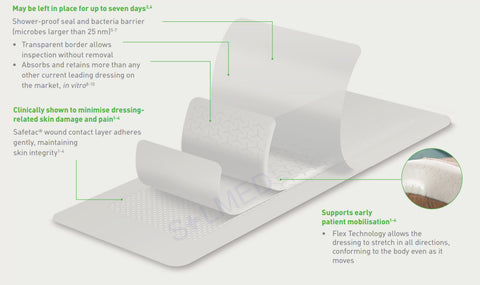
- High tensile strength to enable dressing removal in one piece
- High retention capacity to prevent leakage and maceration
- Absorbs and retains exudate, blood and bacteria
- Highly absorbent, also under compression therapy
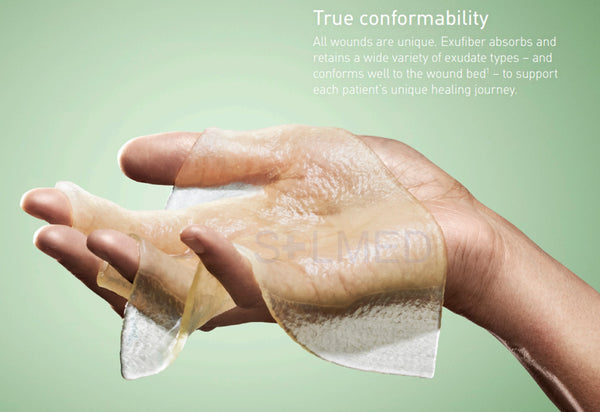
- Soft and conformable which makes it easy to apply
- Hydrolock Technology
- Can be left in place for up to seven days and up to 14 days on donor sites
How does it work?
The soft and conformable non-woven polyvinyl alcohol fibres transform into a gel upon contact with exudate. With hydrolock technology the tightly packed fibres absorb and swell when in contact with fluid, keeping exudate locked in, while the capillary action enables transfer of exudate to the secondary dressing. Exufiber promotes autolytic debridement and is removed in one piece, therefore supporting a clean wound bed.
Areas of Use
Exufiber wound dressings are intended to be used on a wide range of exuding and cavity wounds, such as:
- Leg and foot ulcers
- Pressure injuries
- Partial-thickness burns
- Surgical wounds
- Donor sites
- Malignant wounds
Precautions
Exufiber is not intended for dry wounds or full-thickness burns. If the dressing dries out and is difficult to remove, it should be moistened according to the local clinical practice and allowed to soak until it lifts easily.
Resources
Returns Policy
There is a 30 day return on any faulty products. To be eligible you must return items at your cost. We guarantee that any product found to be faulty supplied by us, will be accepted for return and we will refund all costs associated with the returned item. Statutory Warranties and conditions of Sale apply in Australia under consumer law for all products supplied by Australian Companies.
IMPORTANT NOTE: Should any faulty item be a TGA Registered Medical Product or Device, we are required to submit a report to the Registered Manufacturer in accordance with the TGA Legislation. This is a way of ensuring that any device is safely tracked and monitored for safety. We may ask you a few questions and we are required to verify any fault. All standard State & Federal Warranty Conditions apply.
Shipping
SOLMED only supplies to Australian Addresses.
Solmed dispatches all goods via Australia Post or TNT whichever is deemed suitable. We post to all addresses in Australia including Post Office Boxes. Tracking will be notified on Parcels where applicable. Solmed will advise if any product is not available and offer either a substitute, refund or back order.
Related Products
$7.00